We sat down with some of the original team members from Schrödinger and Nimbus Therapeutics who led the tyrosine kinase 2 (TYK2) inhibitor program that resulted in NDI-034858, an oral, selective allosteric inhibitor being evaluated for the treatment of multiple autoimmune diseases following successful recent Phase 2b results in psoriasis. On December 24, 2022, Takeda entered into an agreement to acquire Nimbus Lakshmi, Inc., a wholly-owned subsidiary of Nimbus Therapeutics, and it’s TYK2 inhibitor, NDI-034858, in a $6 billion deal, with $4 billion up front. Here they discuss the challenges they faced and how these were overcome to successfully develop a potential best-in-class medicine – because they had the right vision and mindset to do things very differently.
When Atlas Venture partner Bruce Booth and Schrödinger’s CEO Ramy Farid co-founded Nimbus Therapeutics in 2009 (then called Project Troubled Water in reference to WaterMap), they took a very different approach to traditional drug discovery. By deploying Schrödinger’s computational platform at scale, they took the risk of relying on physics to guide their decision-making, and bypassed much of the trial-and-error experimentation that is the basis of typical drug discovery.
To implement this approach, Nimbus and Schrödinger scientists operated as one team. This approach required strong collaboration not only between the two teams, but also between medicinal chemists and computational chemists (modelers) who usually work in separate silos throughout the industry. This alignment offered a competitive advantage for the new company.
“The tight integration of the computational and medicinal chemistry teams was critical to the success of the TYK2 effort,” said Craig Masse, senior vice president of discovery research at Ajax Therapeutics, and former executive director of medicinal chemistry at Nimbus Therapeutics. “Our project teams consisted of Schrödinger scientists working closely alongside Nimbus medicinal chemists to produce bespoke solutions and develop new in silico algorithms to overcome program challenges.”
The team also had to commit to allowing computation to drive drug design, which required trust in the technology. The decision to synthesize compounds for preclinical testing was going to be primarily informed by modeling predictions – a significant move away from decision-making approaches in traditional compound design projects.
“Nimbus succeeded where other companies have not because Bruce Booth shared our vision to deploy computational technology at scale and he empowered the team to be guided by the technology,” said Ramy Farid, chief executive officer at Schrödinger. “This was considered revolutionary in drug discovery.”
Nimbus identified drug targets based on established genetic and/or clinical validation, availability of protein structures, amenability to Schrödinger’s computational platform, and ability to deliver value to patients with unmet medical needs. In 2011, tyrosine kinase 2 (TYK2) met all these criteria.
A proven platform, and a critical juncture ahead
TYK2, a member of the Janus Activated Kinase (JAK) family, regulates the signaling of pro-inflammatory cytokines, including interleukin (IL)-23, IL-12 and Type 1 interferon (IFN). Genetic data strongly links TYK2 with immune-mediated diseases, and TYK2 inhibitors have shown promise for the treatment of multiple immune-mediated disorders, including rheumatoid arthritis, psoriasis, inflammatory bowel disease, and lupus.
Each member of the JAK/TYK family contains a kinase domain (JH1) and a pseudokinase domain (JH2). JAK inhibitors target the catalytic site in the JH1 domain because this region is responsible for its kinase activity; however, the challenge is that this region is nearly identical across all four family members, making it difficult to identify selective inhibitors. For this reason, all currently FDA-approved JAK inhibitors are associated with off-target side effects associated with non-selective inhibition of other members of the JAK family. The initial design challenge for the Schrödinger-Nimbus team was to develop a highly selective and potent development candidate that targeted the JH1 domain of TYK2 with a goal of maintaining or improving clinical activity while avoiding off-target side effects mediated by inhibition of other members of the broader enzyme family.
TYK2, JAK1, JAK2, JAK3 superimposed in complex with tofacitinib shows extremely high similarity in the active site – to achieve selectivity required accurate modeling of ligand binding to all four proteins
From hit discovery through lead optimization, the team used Schrödinger’s physics-based computational platform to discover selective inhibitors against the JH1 domain of TYK2. This was the first time in drug discovery history that free energy perturbation (FEP+) was used at scale in a program. A development candidate targeting the JH1 domain was identified that was highly selective against JAK2 and moderately selective against JAK1 and JAK3.
“This served as a backdrop to demonstrate the advantage of utilizing large-scale physics-based modeling of potency, selectivity, solubility, and permeability to guide day-to-day decision-making around synthesis and ideation,” said Sayan Mondal, senior director, computational chemistry at Schrödinger. “These innovations and learnings would serve us well in the twists and turns this project was about to take.”
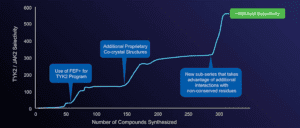
Free energy calculations enabled breakthroughs in achieving selectivity of TYK2 JH1 domain inhibitors
Around the same time that the team identified a development candidate for the JH1 domain of TYK2, a Bristol Myers Squibb (BMS) publication confirmed that highly selective TYK2 inhibitors could be achieved by targeting the allosteric site (JH2 domain).
“This publication provided biological validation that functional activity could be inhibited through the allosteric site,” said Masse. “The team had to make a big decision: should we continue with clinical development of the first development candidate targeting the JH1 domain, or should we completely pivot and target the allosteric site?”
The team chose to pivot.
A quick pivot
The design challenge now was to focus on developing allosteric inhibitors that selectively bind the JH2 domain of TYK2 and disrupt kinase activity.
“We pivoted rapidly to the allosteric site, debuting an innovation in FEP+ modeling to discover picomolar cores in about three months,” said Mondal. “The chase was on! We knew we had developed a potent and selective TYK2 inhibitor once and we could do it again – and we did.”
Overall, more than 13,000 compounds were assessed computationally using FEP+ (which would have required more than five years to do experimentally), allowing the team to profile multiple chemical series simultaneously. The physics-based technology allowed the team to optimize these compounds for drug-like properties while maintaining picomolar potency, eventually leading to the lead clinical compound, NDI-034858.
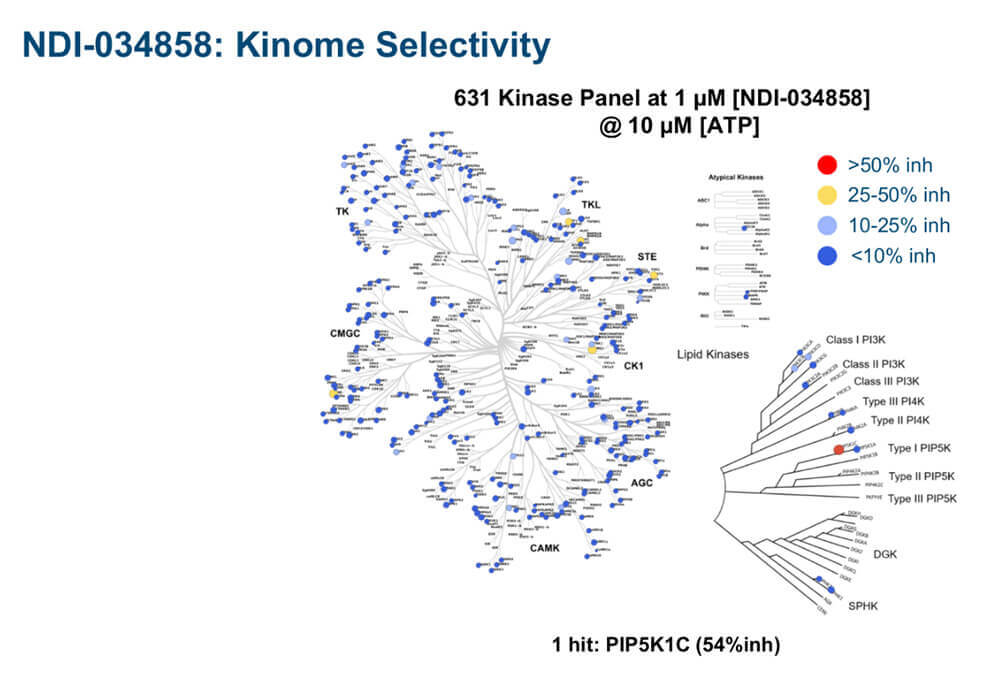
Exquisitely clean selectivity profile of NDI-03458 in a comprehensive 631 kinase selectivity panel. The picomolar inhibitor, which had been tuned precisely to target the JH2 domain of TYK2, spared almost all kinases at 50% inhibition (blue dots) and hit only one kinase at 54% inhibition (red dot) at 1 µM concentration.
NDI-034858 is now being evaluated for the treatment of multiple autoimmune diseases following the recent successful Phase 2b trial in psoriasis. On December 24, 2022, Takeda entered into an agreement to acquire Nimbus Lakshmi, Inc., a wholly-owned subsidiary of Nimbus Therapeutics, and it’s TYK2 inhibitor, NDI-034858, in a $6 billion deal, with $4 billion up front.
New technology, new approach
The TYK2 program laid the groundwork for future innovations to Schrödinger’s physics-based computational platform, including the use of FEP+ at scale. “The advent of state-of-the-art FEP methods has enabled prospective potency predictions to the target of interest with exceptional levels of accuracy, essentially making this technology an ‘in silico’ assay,” said Masse.
The tightly integrative approach to compound design that began with the TYK2 program–between companies and between medicinal chemists and modelers–has been successfully applied to numerous drug discovery programs, both in collaboration with leading pharmaceutical and biotech companies and in Schrödinger’s own proprietary pipeline. Ultimately, it took vision and a change in mindset to transform the drug discovery paradigm, and Schrödinger is now beginning to realize the value that can be created through these efforts.