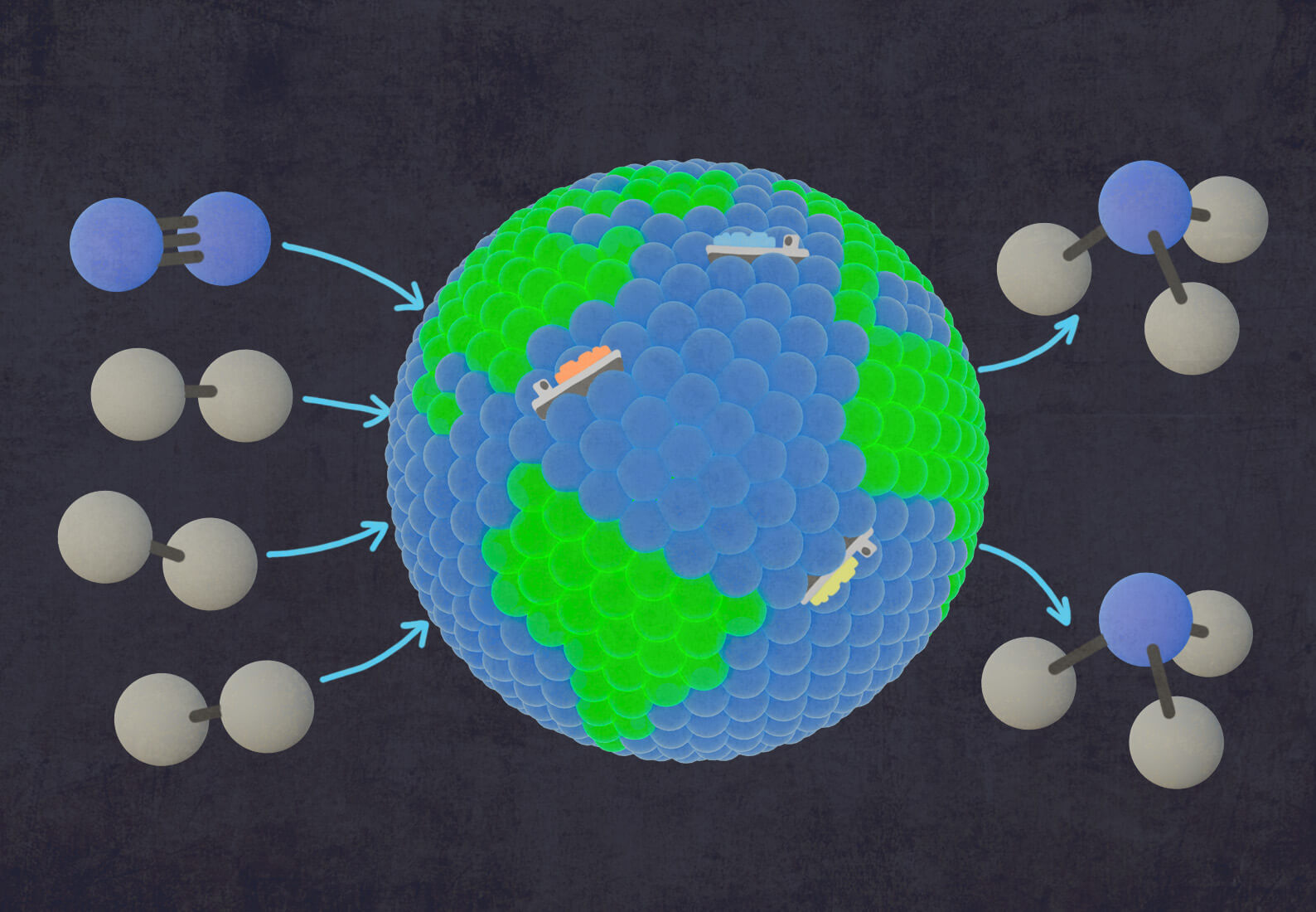
All across the globe, thousands of factories are churning out 180 million metric tons of ammonia each year. This process is highly energy-intensive, generating 1 percent of the planet’s total carbon emissions. It is also essential, as 85 percent of ammonia produced today is used to make synthetic fertilizer, enabling the large-scale crop production that sustains our global food system. Looking forward, sustainable ammonia also has the potential to revolutionize the energy industry, as a zero-carbon shipping fuel, a vector for green hydrogen transport, and for low-carbon electrical power generation.
Underpinning today’s $80 billion worldwide ammonia industry are tiny pebbles a few millimeters in diameter. These pebbles are made up of a class of materials called catalysts, which are often referred to as the ‘workhorses’ of the chemical industry because they facilitate faster, more energy-efficient chemical reactions.
Catalysts play an important role in a wide range of industries, including energy, agriculture, pharmaceuticals, and chemical manufacturing. In the case of conventional ammonia production, nitrogen and hydrogen gasses are combined with an iron-based catalyst inside an ammonia synthesis reactor. The catalyst, activated by high pressure and temperature, accelerates the reaction between hydrogen and nitrogen to produce ammonia.
This process, known as the Haber-Bosch ammonia synthesis reaction, shaped our world by enabling the efficient and economical production of ammonia. Without the ability to synthesize ammonia and produce synthetic fertilizer, the world wouldn’t be able to sustain current crop yields and meet the nutritional needs of our growing global population. But the chemistry on the surface of those tiny ammonia catalyst pebbles hasn’t changed in more than a century. Current ammonia synthesis catalysts rely on incredibly high heat and pressure, which translates to the massive energy consumption and carbon emissions we see from ammonia production plants globally.
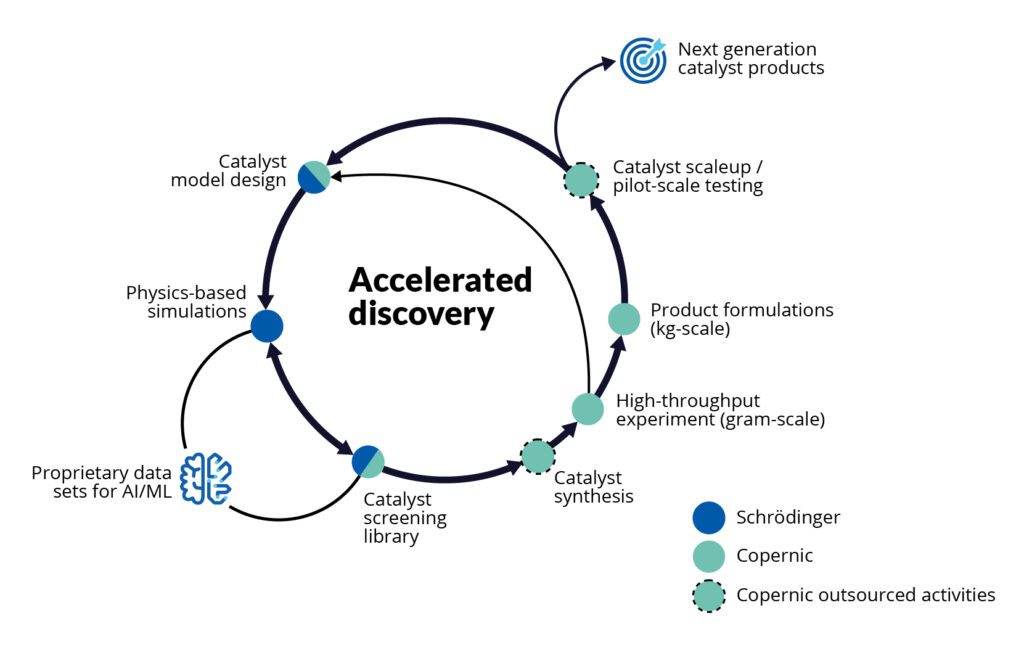
To reduce the carbon footprint and cost of ammonia, we need new catalysts that function more efficiently at lower temperatures and pressures. At Copernic Catalysts, this is our vision. We are developing a portfolio of novel, sustainable catalysts — starting with an ammonia synthesis catalyst — to help the chemical, energy, and agriculture industries reduce reliance on fossil fuels and cut carbon emissions. We’ve partnered with Schrödinger, the global leader in computational molecular modeling, to help us accelerate this work by exploring and optimizing thousands of potential catalyst compounds in silico.
A better ammonia catalyst requires certain properties. To be more sustainable, the novel compound must lead to faster, more energy-efficient chemical reactions. It needs to function with less heat and under less pressure than existing options, and remain stable over the industry standard 10-year lifetime. The replacement material also must be formulated into granules similar in size, shape, and surface area to current catalysts so it can be seamlessly integrated into the global ammonia production infrastructure.
By working with Schrödinger and starting our discovery process with computational screening, we were able to predict, optimize, and rank potential compounds, taking into account all relevant properties, before synthesizing only the best candidates in the lab. This predict-first approach compressed research that would normally take decades into a two-year project. Rather than relying on traditional catalyst development — which involves failing a million times before discovering a promising catalyst — Schrödinger’s physics-based computational platform and modeling expertise allowed us to screen a much wider class of materials than we could have otherwise, and to understand relevant catalyst mechanisms with more clarity and a higher degree of accuracy than we could without computation.
This predict-first approach compressed research that would normally take decades into a two-year project.
The success of our collaboration with Schrödinger has resulted in a breakthrough ammonia catalyst, which we’ve now experimentally validated in our lab, showing that it outperforms the existing iron catalysts by more than 3x under mild operating conditions. This is an exciting step toward reducing the current carbon footprint of ammonia production. Our hope is that by making the production of green ammonia more cost-effective, we can help enable its widespread use in the energy sector in a myriad of applications.
As we work to scale up our lead ammonia catalyst compound, we are simultaneously initiating new discovery work with the Schrödinger team, developing computational models for other novel catalysts critical to sustainability. We recently signed a four-year research collaboration to build on our previous success. Sustainable catalysts can help drive decarbonization in some of the hardest-to-decarbonize industries, and we’re excited to be working with Schrödinger to address this urgent challenge.